The European Union’s new Medical Device Regulation (MDR) that aims to improve clinical safety became fully applicable on May 26. Unlike directives, regulations do not need to be transposed into national law.
The Medical Device Regulation affects all medical devices sold in Europe, including Formlabs products. Our blog post covers frequently asked questions about the new Medical Device Regulation and how it impacts Formlabs and our customers.
What is a medical device?
A medical device is defined as “any instrument, apparatus, appliance, software, implant, reagent, material, or other article” intended to be used for the diagnosis, prevention, or treatment of diseases and injuries.
What Formlabs products are medical devices?
| Medical Device | Class | Manufacturer |
|---|---|---|
| Surgical Guide | Class I | Formlabs |
| Custom Tray | Class I | Formlabs |
| IBT | Class I | Formlabs |
| Dental LT Clear V2* | Class IIa | Formlabs |
| Dental SG | Class I | 3rd party |
| Dental LT Clear V1 | Class IIa | 3rd party |
| Temporary CB | Class IIa | 3rd party |
| Permanent Crown | Class IIa | 3rd party |
| Denture Base | Class IIa | 3rd party |
| Denture Teeth | Class IIa | 3rd party |
*not available yet in Europe
How can I ensure that the Formlabs medical devices I am using are MDR Compliant starting May 26th, 2021?
Class I devices - Changes will only apply to devices manufactured after May 26th 2021.
According to the EU MDR Article 120, Paragraph 4, devices lawfully placed on the market pursuant to Directive 93/42/EEC (MDD) prior to 26 May 2021 may continue to be made available on the market or put into service until 27 May 2025.
Therefore, Formlabs certifies that its Class I medical devices manufactured before May 25th, 2021, and expiring no later than May 25th, 2023 can be used and sold in accordance with the EU MDR.
Download the Official Statement.
Class IIa devices - No changes until 2024* (check the official statement for the exact dates)
According to the EU MDR Article 120, Paragraph 4, devices lawfully placed on the market pursuant to Directive 93/42/EEC (MDD) by virtue of a valid certificate (valid until the end of the period indicated on the certificate) may continue to be made available on the market or put into service until 27 May 2025.
Therefore, Formlabs certifies that its Class IIa medical devices can be used and sold in accordance with the EU MDR.
For medical devices produced by Formlabs after May 26th, 2021, what changes will be visible on the labels?
Labeling includes the IFU, back label, and any other label placed on the cartridge or box. MDR labeling is different from MDD labeling in that it should include:
-
An "MD" symbol (signifying the product is a medical device)
-
Importer name
-
UDI (unique device identification)
-
IFUs with clinical benefits, residual risks, and other Annex I requirements
Formlabs products will feature MDR compliant labels (if applicable). Note that just because labeling includes all of the above doesn't mean that the medical device in question meets all MDR requirements.
What certifications are needed?
Class I devices don’t require certification, only a Declaration of Conformity (DoC), which constitutes as the manufacturer’s self-certification. These devices still must be manufactured and labeled according to the regulation.
Class IIa devices require an EC Certificate issued by a Notified Body and also require a Declaration of Conformity (DoC).
What do these changes mean for Formlabs?
These changes present different meanings for Formlabs devices.
Formlabs Class I devices
-
Class I devices will switch from being MDD products to MDR products, once the stock has been depleted.
-
Formlabs will have an MDR DoC.
-
Products will have MDR-compliant labels and IFUs.
Formlabs Class IIa devices
-
When Dental LT Clear V2 becomes available in Europe, it will have an MDR EC Certificate and Formlabs’ quality system will be MDR certified.
3rd party Class IIa devices
-
Class IIa devices will maintain an MDD certificate and no changes will be made until that certificate expires (dependent on the certificate, no later than May 26th 2024)
By when do we expect Formlabs MDR declaration of conformity (DoC)?
All Class I medical devices distributed by Formlabs and manufactured after May 26, 2021 need a new DoC. Formlabs Class I devices will have an MDR DoC for the batches manufactured after May 25, 2021
Does the MDR DoC apply to resins and printers?
MDR DoC applies to all Class I resins Formlabs sells. However, MDR does not apply to Formlabs printers. Printers have a DoC for different legislation.
What are the requirements for Formlabs dental customers?
Under MDR, dental labs and practices are now manufacturers. Any product that constitutes a medical device must be made under an established quality system.
Manufacturers must:
-
Meet all applicable items from the General Safety and Performance Requirements
-
Comply with all post-market requirements
-
Register as a device manufacturer (territory specific)
-
Write up an Annex XIII statement (similar to a DoC)
-
Appoint a person responsible for regulatory compliance
How can Formlabs help customers meet their MDR obligations?
Formlabs can help customers meet the GSPR (General Safety and Performance) Requirements (MDR Annex I) by providing on request:
-
Certificate of Analysis ⇒ Shows batch control and quality
-
Certificate of Compliance ⇒ Shows conformance to Annex I
Other documents to support compliance:
-
Declaration of Conformity of medical devices - download documents at the “Downloads” section for each indication (if applicable)
-
TDS - download documents at the “Downloads” section for each indication
-
SDS - download documents at the bottom of the page
-
Proof that the Class I devices are MDR compliant (manufactured before May 26th)
By tracking equipment and materials, Formlabs’ online Dashboard helps with the documentation requirements of Article 5.
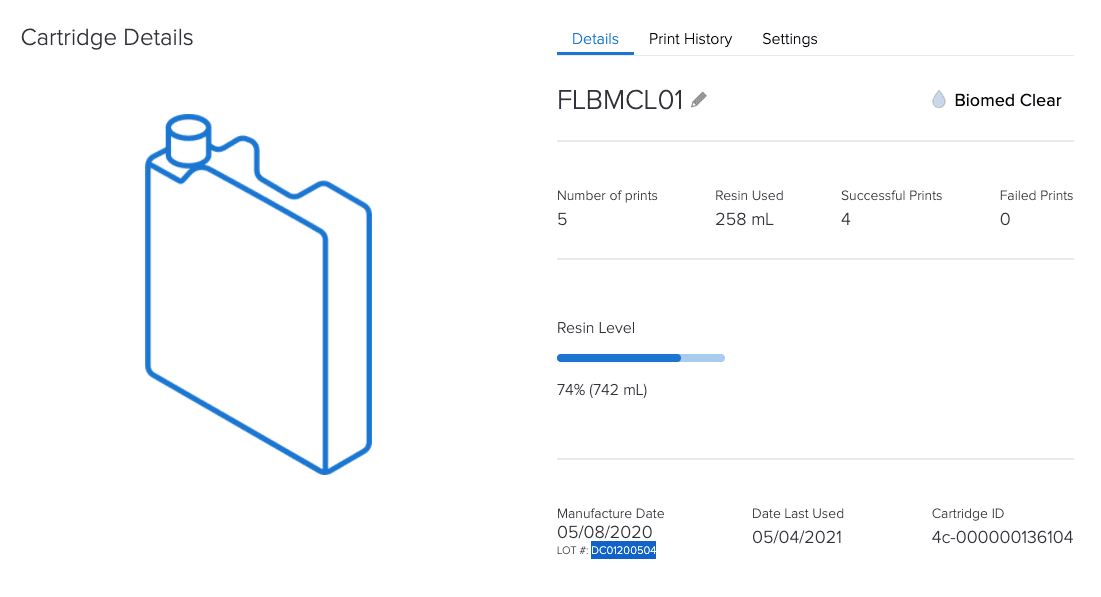
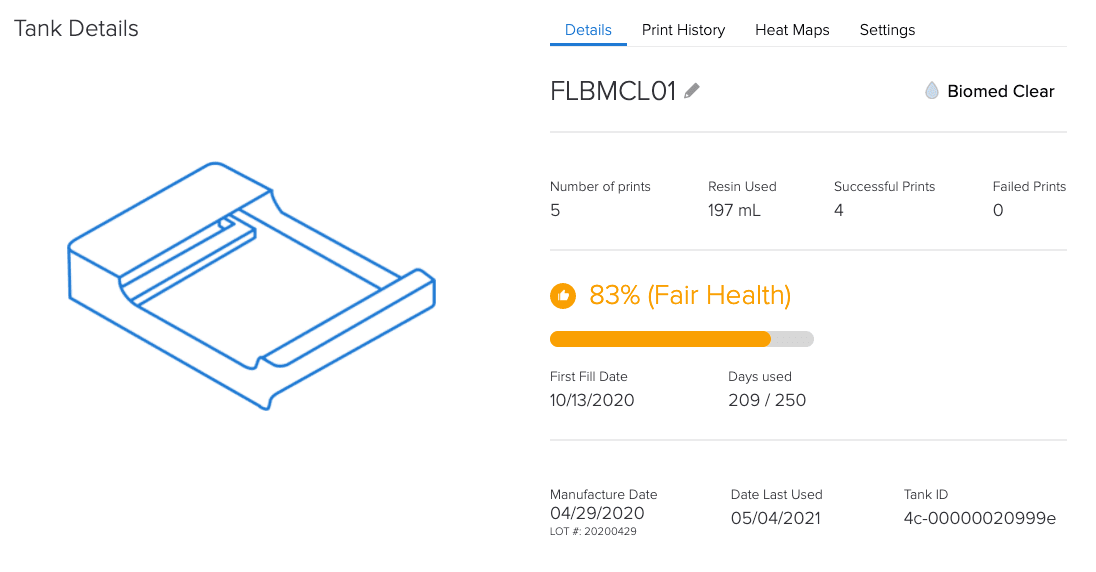
How is a Formlabs controlled ecosystem an advantage under MDR?
Since all dental customers (labs and practices) and medical institutions have to comply with all MDR requirements, having a controlled system can provide confidence and compliance.
-
A controlled system allows for complete Annex I compliance assurance for the printer and material.
-
A clinical evaluation supporting the material and printer.
-
Compliance in the form of documentation for each batch and digital documentation.
Formlabs printers, materials, and associated products comply with a variety of safety and quality standards in different jurisdictions. You can find more information here.
Learn More About MDR
Watch our webinar about MDR where Sam Murray, Director of Regulatory Affairs and Quality Assurance at Formlabs provides a detailed overview of the new regulation and how it affects Formlabs customers.