
Implementing 3D printing workflows in your lab or practice can be a game changer for your business. However, with so many solutions now available, there can be a lot to weigh when selecting a 3D printer and materials, including regulatory implications. Understanding the basics of regulatory compliance when it comes to dental 3D printers, materials, and closed versus open ecosystems can help inform the risks you take when 3D printing dental appliances and parts.
Regulation depends on geography. Here, we examine regulations in the United States of America and the European Union when it comes to using different resins classified as medical devices on 3D printers.

Get in Touch
Whether you need to produce occlusal splints in-house, or are looking for high-throughput production of dental models, we're here to help. Get in touch with a dental expert to find the right solution for your business.
Introduction
3D printing is gaining popularity among dental professionals, fueling the development of dental 3D printing solutions and materials. According to a 2023 survey by the Journal of the American Dental Association (JADA) 17% of respondents currently use a 3D printer. Labs have an even higher adoption rate. A 2024 survey of more than 215 dental labs in the US by Key Group Inc. found that 60% (up from 45% in 2020) of dental labs in the United States have 3D printing technology and 66% are planning to purchase or lease a 3D printer in the next 12 months.
With so many solutions available, there can be a lot to weigh when selecting a 3D printer and materials, including regulatory implications. Understanding the basics of regulatory compliance when it comes to dental 3D printers, materials, and closed versus open ecosystems, can help inform the risks you take when 3D printing dental appliances and parts.
What Is a Medical Device?
One of the first things to determine when using 3D printing in your dental business is if you are using a medical device. Some 3D printer resins are categorized as medical devices in the dental space. This means a one-liter cartridge of resin can be a medical device based on how it is regulated.
In the United States, per Section 201(h)(1) of the Food, Drug, and Cosmetic Act, a device is:
An instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory which is:
A) recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them,
B) intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or
C) intended to affect the structure or any function of the body of man or other animals, and which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of its primary intended purposes. The term "device" does not include software functions excluded pursuant to section 520(o).
In the European Union, per the Medical Device Regulations (MDR), “medical device” means any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes:
-
diagnosis, prevention, monitoring, prediction, prognosis, treatment or alleviation of disease, diagnosis, monitoring, treatment, alleviation of, or compensation for, an injury or disability,
-
investigation, replacement or modification of the anatomy or of a physiological or pathological process or state,
-
providing information by means of in vitro examination of specimens derived from the human body, including organ, blood and tissue donations,
and which does not achieve its principal intended action by pharmacological, immunological or metabolic means, in or on the human body, but which may be assisted in its function by such means.
Examples of Dental 3D Printing Resins Classified as Medical Devices

Resin for 3D printing flexible occlusal splints, occlusal guards, and bleaching trays

Resin for 3D printing hard occlusal splints and night guards

Resin for 3D printing dental surgical implant guides

Premium Teeth Resin
Resin for 3D printing denture teeth and full-arch implant-supported restorations (All-on-X appliances), temporary single units (crowns, inlays, onlays, veneers), and temporary bridges up to seven-units
Examples of Dental 3D Printing Resins That Are Not Medical Devices
Resin for 3D printing dental models for thermoforming
Similar to other medical device manufacturers, manufacturers of dental 3D printing resins that are classified as medical devices are ultimately responsible for their material. This is no different than the responsibilities of any other medical device manufacturer. These responsibilities include the design and development work along with appropriate manufacturing to ensure that the device will meet its intended purpose.
In 3D printing, these responsibilities include:
-
Developing the correct resin formula
-
Creating appropriate print settings
-
Verifying the print process for accuracy and mechanical properties
-
Ensuring biocompatibility, either through testing or rationalization, depending on the device categorization per ISO 10993-1
-
Providing necessary labeling for how to use the product
Additionally, medical device manufacturers are responsible for following the appropriate regulatory registrations in all the jurisdictions in which their materials are being sold. Based on the classification of the product, this can include a 510(k) clearance from the US FDA, or CE Marking for the EU.
A 510k is a submission to the FDA, where a manufacturer is trying to demonstrate substantial equivalence between their device and one already legally marketed. This means showing that the two devices are equivalent in terms of intended use, performance testing, technological characteristics, etc.

Product Demo: Form 4B Dental 3D Printer
Blazing speed meets unmatched accuracy in the next generation of dental 3D printing. Explore the latest innovations in our webinar.
MDR in the European Union
Within the European Union, under the requirements of the Medical Device Regulation (MDR), dental and orthodontic labs and practices are considered medical device manufacturers. Any product that constitutes a medical device must be made under an established quality system.
Under MDR, manufacturers must:
-
Meet all applicable items from the General Safety and Performance Requirements
-
Comply with all Post-Market Requirements appropriate for device type and risk classification. This is a continuous process of monitoring and collecting data on the safety and performance of medical devices, which may include trend reporting, complaints analysis, and post-market clinical follow-up.
-
Register as a device manufacturer (territory-specific)
-
Write up an Annex XIII statement (similar to a Declaration of Conformity)
-
Appoint a person responsible for regulatory compliance
The implementation of MDR has changed how dentists and dental labs operate in the EU. However, this transition and its implications have not been clearly communicated to all dental practitioners. Additionally, the various implementation delays have added to the confusion about the requirements and deadlines that must be met.
“Even though MDR has been in effect for some time, there’s still a lack of complete clarity about the full extent of its implications.”
Stephan Kreimer, MDT, Kreimer Dentallabor
There are different levels of regulatory risk based on geographic location. In the United States, there is more leeway given to medical practitioners to do what they think is best for their patients. In the EU, a medical practitioner would still be held to the requirements of the EU MDR. As you deviate from the resin manufacturer’s instructions, whether that be print settings, post-processing, or indications for use, you take on more risk of the medical device.

Formlabs Dental Materials Overview: Discover What You Can Print With the Form 4B
This webinar will guide you through the various benefits and applications enabled by Form 4B, including its existing biocompatible materials and newly developed formulations for model production such as Fast Model Resin and Precision Model Resin.
The Spectrum of Risk: US Labs and Practices
For dental professionals using medical device resins to print dental appliances, the tools, materials, and workflows followed exist on a spectrum of risk. Some choices of 3D printing ecosystem and materials won’t add any risk to a business. Other choices can shift the risk from the resin manufacturer to the business or user doing the 3D printing.
Below, we outline common scenarios and the level of regulatory risk the user takes on for each.
Medical Device Resin From the 3D Printer Company
Scenario: A user is printing on a 3D printer with a resin that is manufactured by the same manufacturer as the printer. This is a totally closed ecosystem in which the manufacturer has control over both materials and printers. The user follows all instructions provided by the manufacturer.
Regulatory Risk for the User: Low
Scenario: A user is printing on a 3D printer with a resin that is manufactured by the same manufacturer as the printer. This is a totally closed ecosystem in which the manufacturer has control over both materials and printers. However, the user is not following all the instructions provided by the manufacturer and is instead modifying the workflow in some way. Examples of modifying the workflow include, but are not limited to, utilizing different post-processing instructions or changing recommended print settings.
Regulatory Risk for the User: Moderate
Depending on the changes made to the workflow, the user is taking on some regulatory risk.
Medical Device Resin From a Non-3D Printer Company: Third-Party
Scenario: A user is printing on a 3D printer with a resin that is manufactured by a third party, but the third party has validated the print settings and workflow on the selected 3D printer. The user follows all instructions provided by the resin manufacturer.
Regulatory Risk for the User: Low to moderate
Yes, the workflow has been validated and the third-party resin manufacturer has done the appropriate regulatory work, but if the relationship between the resin manufacturer and the printer manufacturer is unknown, some risk is being taken by the user. For example, there could be changes to the firmware, software, or even the printer itself that the resin manufacturer was not made aware of. These changes could impact the performance of the material or printed part.
Scenario: A user is printing on a 3D printer with a resin that is manufactured by a third party, but the third party has validated the print settings and workflow on the selected 3D printer. However, the user is not following all the instructions provided by the manufacturer and is instead modifying the workflow in some way. Examples of modifying the workflow include, but are not limited to, utilizing different post-processing instructions or changing recommended print settings.
Regulatory Risk for the User: Moderate
The same risks apply as with using any third-party resin, but the risk is increased any time the prescribed workflow is modified.
Off-Label Usage of a 3D Printer Resin
Scenario: Regardless of whether the material was part of an open or closed printer ecosystem, the user is choosing to manufacture a dental application that is not specifically indicated for use by the resin manufacturer.
Regulatory Risk for the User: Moderate to high
Deviating from the intended use or specific indications of use provided by the manufacturer adds user risk. Using a resin for an indication that has not been validated by the manufacturer and has not gone through the appropriate regulatory processes means taking on risk.
Scenario Summary
| Resin Indicated for Dental Application | Resin from 3D Printer Company | User Using Validated Workflow | Regulatory Risk for the User |
|---|---|---|---|
| ✓ | ✓ | ✓ | Low |
| ✓ | ✓ | X | Moderate |
| ✓ | X | ✓ | Low to moderate |
| ✓ | X | X | Moderate |
| X | X | X | Moderate to high |
There are different levels of regulatory risk based on geographic location. In the United States, there is more leeway given to medical practitioners to do what they think is best for their patients. In the EU, a medical practitioner would still be held to the requirements of the EU MDR.
As you deviate from the resin manufacturer’s instructions, whether that be print settings, post-processing, or indications for use, you take on more risk of the medical device.
Formlabs’ Approach to Dental Resins
Formlabs chooses to address the dental resin market with three unique offerings:
-
In-house materials that Formlabs develops: 15+ dental resins for a range of applications
-
Certified third-party materials: When an existing resin on the market is identified that fills a gap in our portfolio we work closely with the manufacturer to ensure the material works appropriately on our printers.
-
Open Material Mode: Allows a user to print with any 405 nm photopolymer resin on a Formlabs 3D printer. This product provides expert users with full access to third-party materials, empowering them to explore endless new possibilities. The resin manufacturer is ultimately responsible for the product and any regulatory certifications that are needed.
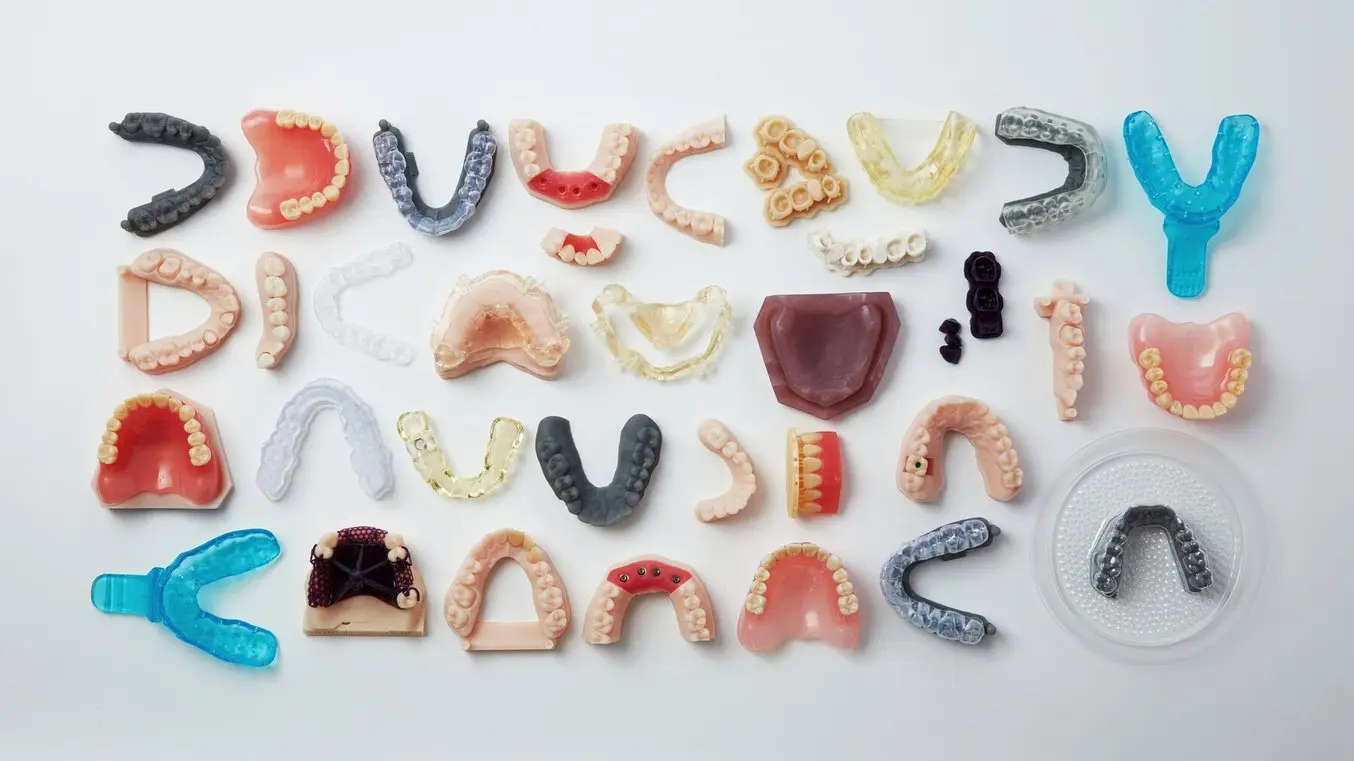
The Formlabs Dental materials library includes 15+ resins for a range of applications.
The approach Formlabs takes allows our users to choose what is best for their business. Many printer ecosystems are only open or closed; Formlabs’ unique platform blends the best of both models to provide one solution for all your printing needs.
“Nowadays in Europe, there is very strict regulation of 3D printing medical devices, the MDR. I prefer to print with Formlabs because the workflow is validated and the resins that will be printed are certified.”
Dr. Antonino Cacioppo, DDS, PhD, Prosthodontist
The most common dental resins in the Formlabs portfolio are those that are developed in-house. With a team of over 50 material experts, we have positioned ourself at the forefront of materials development. Materials developed by Formlabs go through a rigorous design and development process. Once a formula is developed, verification testing ensures that the resin can meet the mechanical properties needed for the specific intended applications. Additionally, we test for various levels of biocompatibility based on the intended applications or indications of the material.
All of this work, including the manufacturing of the resin, is done through our quality management system that is ISO 13485 and EU MDR certified. Formlabs also handles all of the regulatory registration requirements to be able to sell the product into the market.
Formlabs medical device resins are manufactured at our FDA-Registered facility in Ohio, where a dedicated team of operators and quality assurance professionals make the resins inside a certified ISO Class 8 clean room. These materials cover a range of applications, and technical data sheets and workflow guides can be found on our website.
Request a Free Sample Part
See and feel Formlabs quality firsthand. We’ll ship a free 3D printed sample part printed on the Form 4B to your office.
Certified Third-Party Materials
BEGO™ VarseoSmile® TriniQ® Resin is an example of a certified third-party material and is indicated for temporary and permanent single units (crowns, inlays, onlays, and veneers), bridges, and denture teeth.
As explained above, using the proper workflows with certified third-party materials is of low to moderate risk, as the printer manufacturer and material manufacturer communicate any changes that may affect the workflow or final outcomes.
When an existing resin on the market is identified that fills a gap in our portfolio — for a specific application or geographic location — we work closely with the manufacturer to ensure the material works appropriately on our printers. This certification process involves making sure that we are able to provide print settings that satisfy the requirements of the manufacturer.
The manufacturer tests their product on Formlabs printers and may conduct additional verification and validation tests. Additionally, if any significant changes to hardware are made, these are communicated to the resin manufacturer to ensure the material will still print appropriately.
In these cases, the resin manufacturer is ultimately responsible for the product and any regulatory certifications that are needed.
Open Material Mode

Learn how three dental professionals are using OMM in this article.
Open Material Mode allows a user to print with any 405 nm photopolymer resin on a Formlabs resin 3D printer. This product provides expert users with full access to third-party materials, empowering them to explore endless new possibilities.
Some third-party resin companies will develop their own settings to enable their materials to be printed on Formlabs printers. These third-party resin companies may also conduct additional verification and validation tests to ensure their devices print appropriately on Formlabs printers. The resin manufacturer is ultimately responsible for the product and any regulatory certifications that are needed. Please refer to the documentation provided by these resin manufacturers.
Understanding Your Risk
“We only use biocompatible and certified systems because we want to be ready for regulations. With a certified workflow, we can ensure the quality of our printings. Plus, biocompatibility testing ensures that we are prioritizing patient safety.”
Dr. Édouard Lanoiselée, DDS, Cabinet dentaire de Nozay
When printing with materials considered medical devices, dental and orthodontic labs and practices take on varying degrees of regulatory risk. By understanding the implications of what is being printed, dental professionals can better serve their patients and grow their business.
To evaluate Formlabs Dental resins, request a free sample part or find a reseller to learn more about Formlabs solutions, our comprehensive materials library, validated materials, and Open Material Mode. If you have questions about regulatory risk, schedule an appointment with our regulatory affairs and quality assurance (RAQA) team.